Mecatheque
Results of collective works
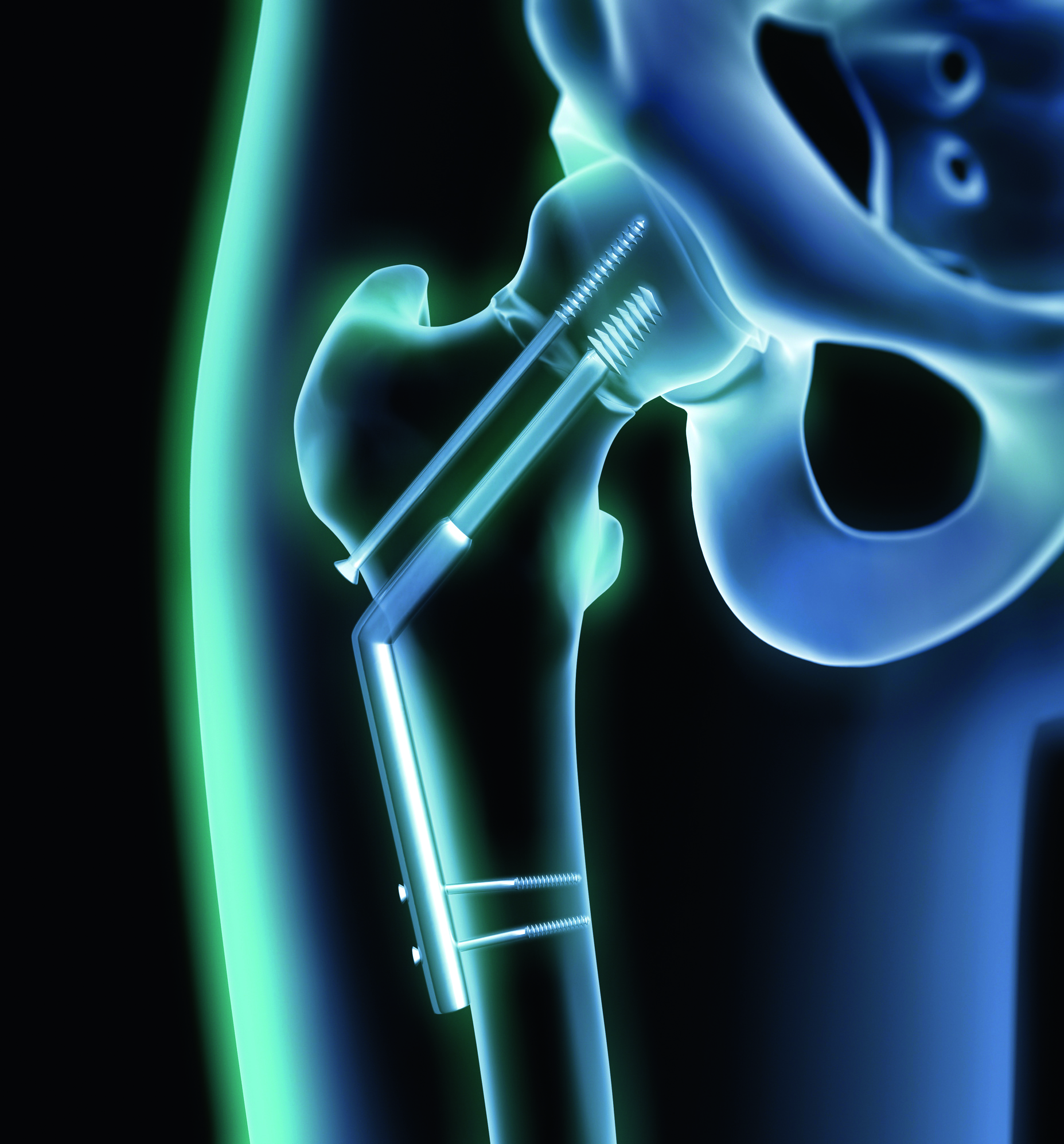
Control of subcontracting within the medical devices sector - Practical guide - 2020 Edition
The ever increasing changes in the expectations and needs of the medical profession and of patients tends to lead to more complex medical devices, the design and manufacture of which involve a number of processes and technologies. This requires specialist know-how and production techniques that the manufactures of these products do not always possess. In order to address this issue, they subcontract the manufacture of all or part of the device, including the design (testing, etc.) and manufacturer, and even the logistics (transport, storage, installation, etc.). In May 2020, regulation (EU) 2017/745 on medical devices will enter into force. One of the clauses of this regulation stipulâtes that the manufacturer’s quality management system must cover the selection and control of subcontractors, who may be subject to audits and inspections by notified bodies as well as the competent authorities. Manufacturers will retain full responsibility for the subcontracted activities. This guide is therefore intended to help them define the measures to be taken to comply with this new regulation via a methodology based not only on this regulation but also on the provisions of standard ISO 13485 which specifies the requirements for a quality management system using a risk-based approach.. The guide (9Q383) therefor addresses and illustrates, though examples, all the issues inherent in the control of the process, from subcontractor selection through to the monitoring methods implemented and applied by the manufacturer.
Author : Benoit Duchazeaubeneix, Cetim



Log in to download
9Q383 - Control of subcontracting within the medical devices sector - Practical guide - 2020 Edition








